Looking for Remote Work?
Remote-First Recruiting
Team Cronos is a remote-first recruiting firm that understands the value that you can bring to an organization. Our recruiters have first-hand experience in their practice areas as engineers, developers, marketers, and analysts. We know what it’s like to be you because we’ve been you.

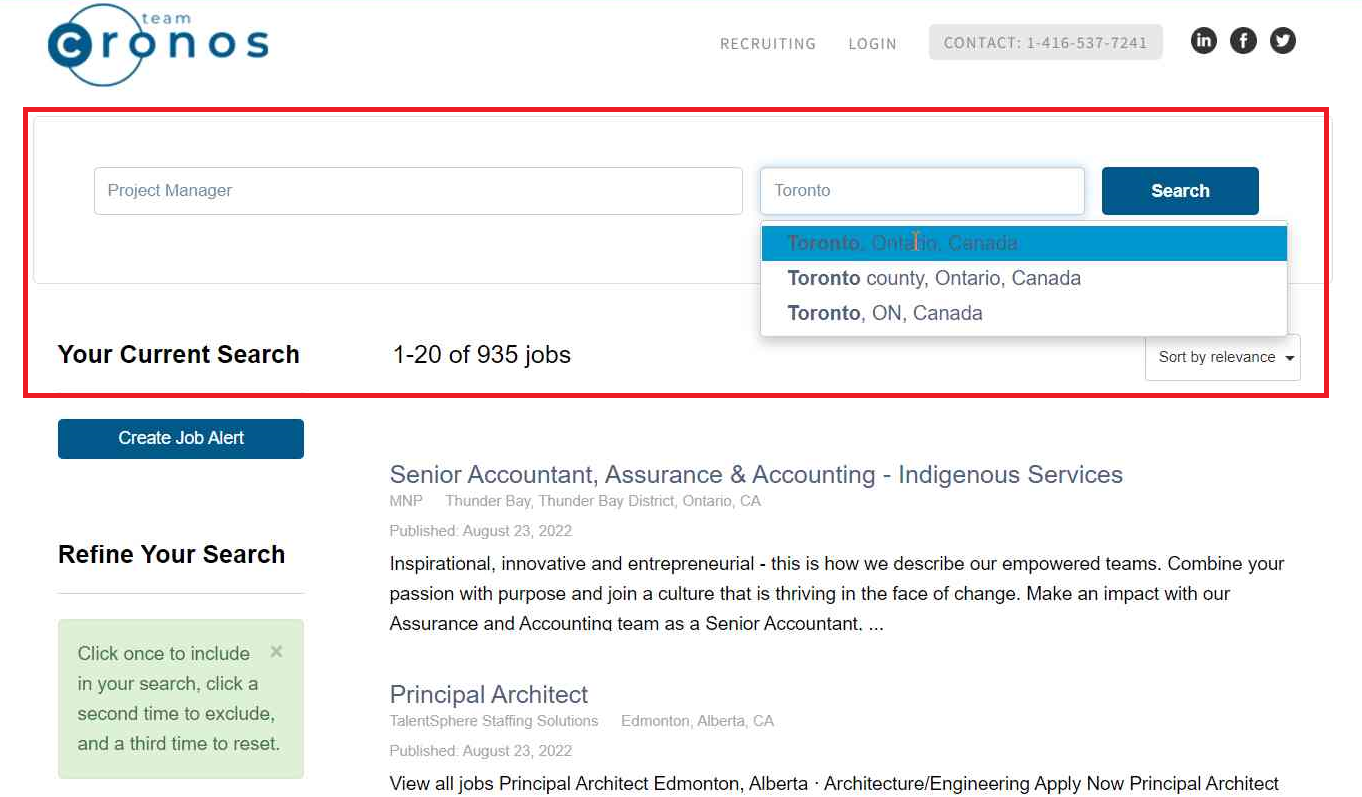
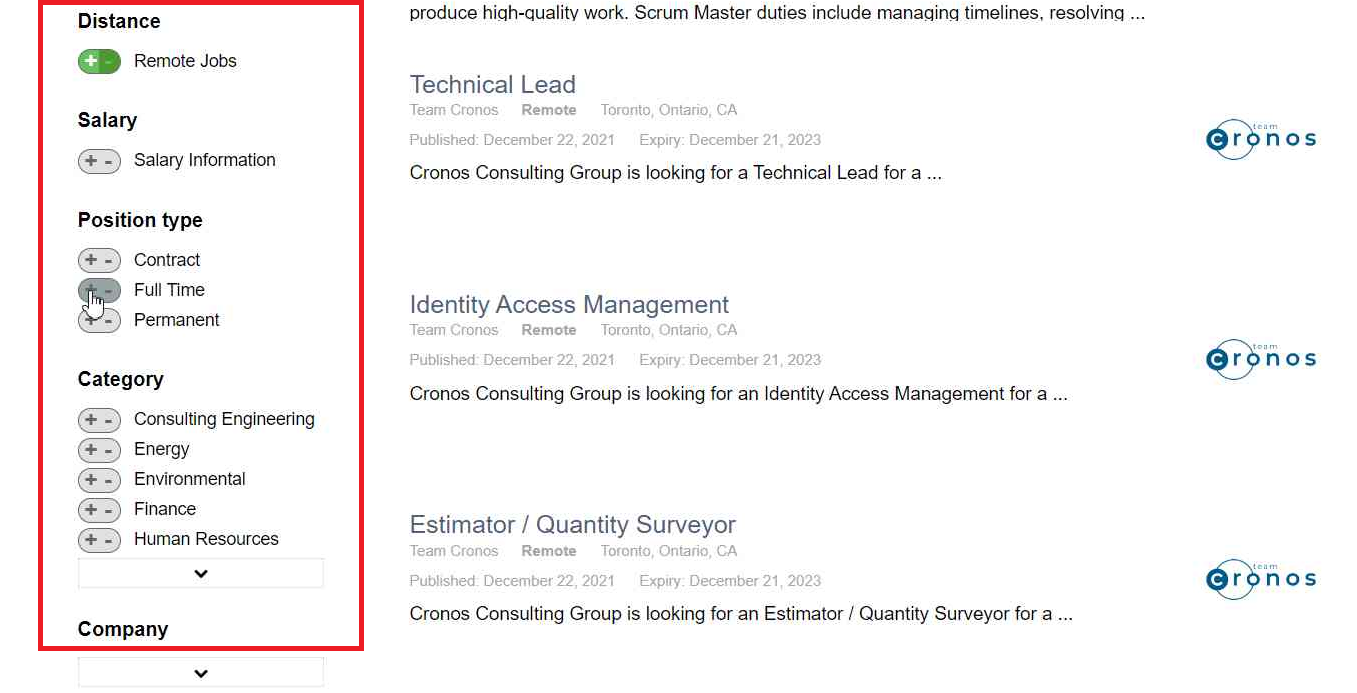
Find the right jobs on our job board
Create an account on our job board and get access to the best remote jobs in Canada!
- Manage your job search with great tools
- Get your resume to the top of the pile
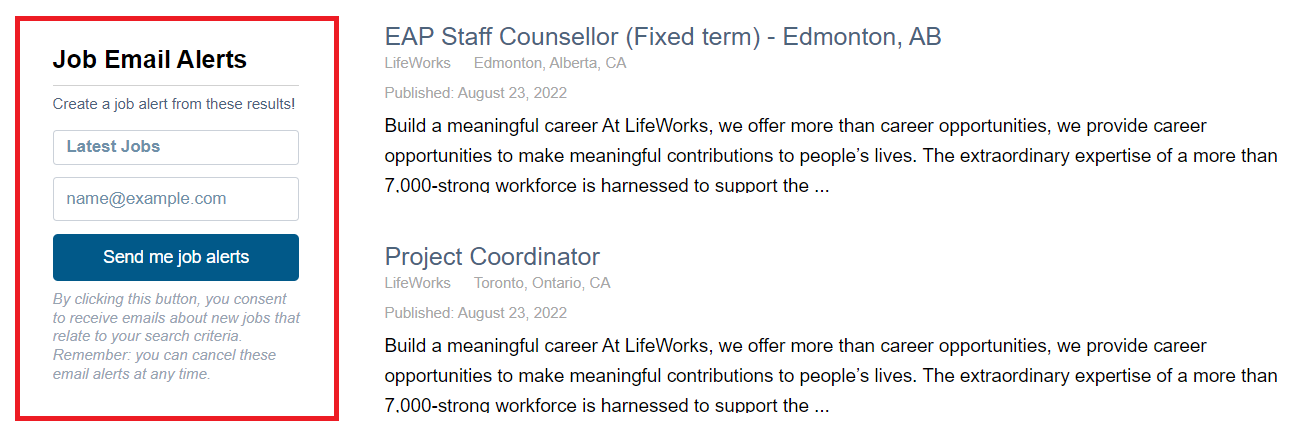
- Sign up for job alerts
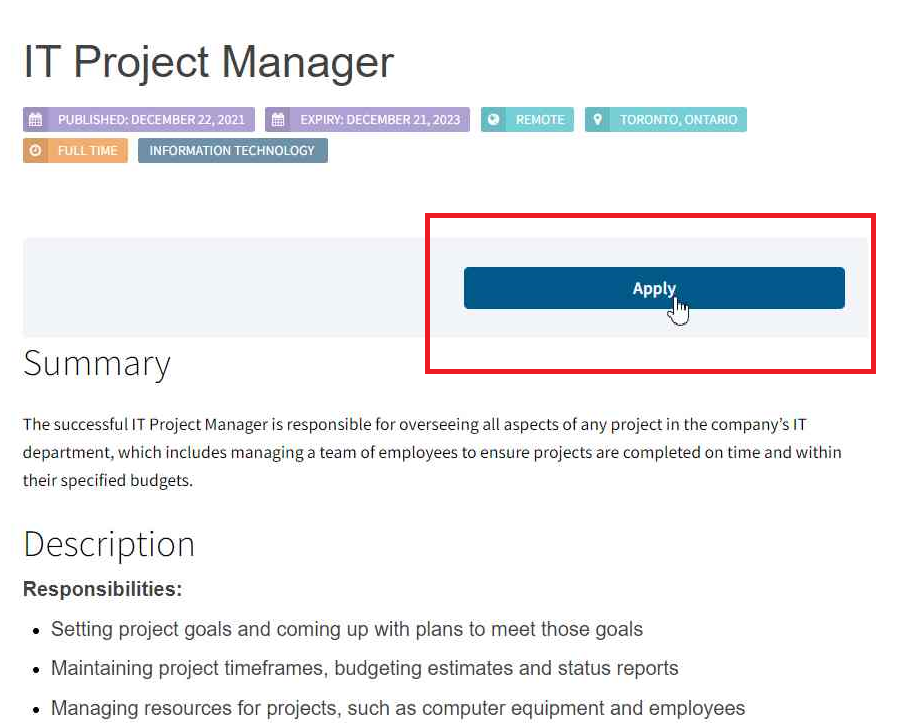
How it works
- Create an account
- Upload your resume and complete your profile
- Talk with one of our recruiters
- Find your next great opportunity